Calculate the 'spin only' magnetic moment of M2+ (aq) ion (Z = 27). - Sarthaks eConnect | Largest Online Education Community

Mn2+–Mn2+ Magnetic Coupling Effect on Photoluminescence Revealed by Photomagnetism in CsMnCl3 | The Journal of Physical Chemistry Letters

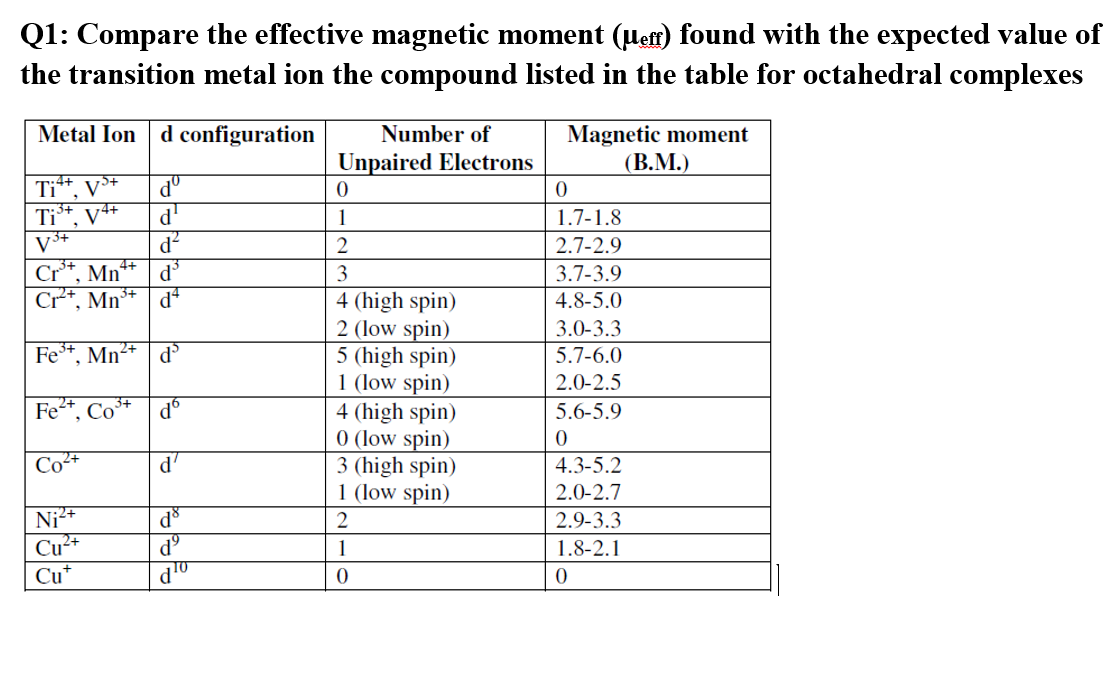
The correct order of spin only magnetic moment (inBM) for Mn^{2+} Cr^{2+} and Ti^{2+} ions is(a} Mn^{2+}>Ti^{2+}>Cr^{2+} (b) Ti^{2+}>Cr^{2+}>Mn^{2+}(c) Mn^{2+}>Cr^{2+}>T^{2+} (d} Cr^{2+}>Ti^{2+}>Mn^{2+} | Snapsolve
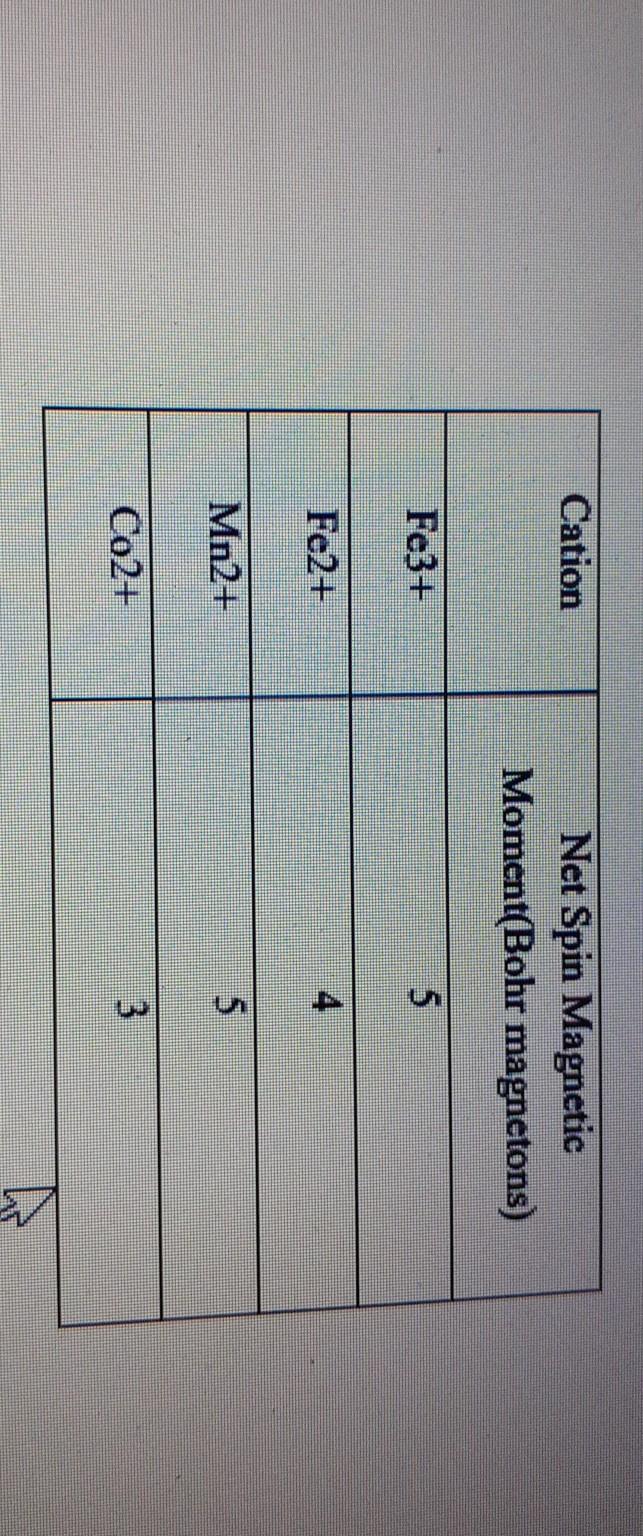
12. Exerimental value of magnetic moment of Mn2+ complex is 5.96BM.This indicates a)axial and orbital motion of electron in same direction b)axial and orbital motion of electron in opposite direction c)electron does

calculate the number of unpaired electrons in Ti3+ , Mn2+ and calculate the spin only magnetic - Brainly.in
The spin only magnetic moment of Tetrachloridomanganate(II)ion is 5.9 BM. On the basis of VBT, - Sarthaks eConnect | Largest Online Education Community

Among V(Z = 23) , Cr(Z = 24) , Mn(Z = 25) and Fe(Z = 26) , which will have the highest magnetic moment?
What is the spin only magnetic moment of Cu+ ion? In my book it's given as root 2? What's the answer? - Quora

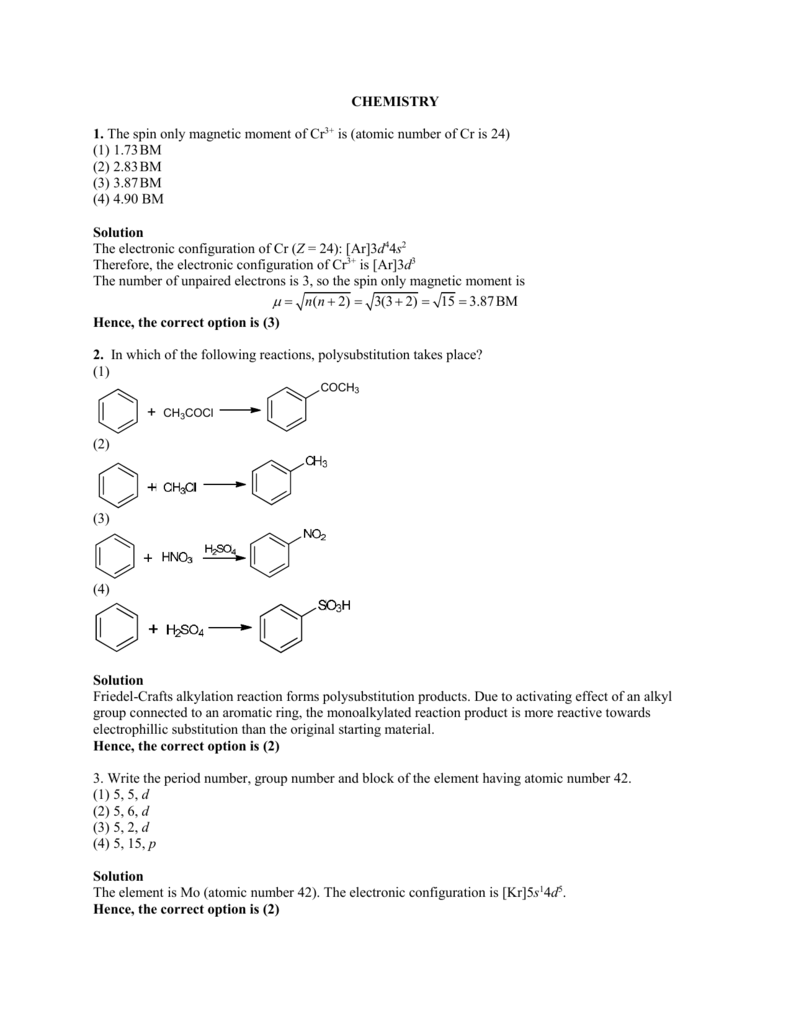
In the fallowing reaction: Cr2O7^2 - (aq) + SO3^2 - (aq) + 8H^+→ 2Cr^3 + + SO4^2 - + H2O the stoichiometric coefficient of SO3^2 - is:
![The spin only magnetic moment value of `[MnBr_(4)]^(2-)` ion is `5.9BM` On the basis of `VBT` Pr... - YouTube The spin only magnetic moment value of `[MnBr_(4)]^(2-)` ion is `5.9BM` On the basis of `VBT` Pr... - YouTube](https://i.ytimg.com/vi/E3pLlLoxf48/maxresdefault.jpg)
The spin only magnetic moment value of `[MnBr_(4)]^(2-)` ion is `5.9BM` On the basis of `VBT` Pr... - YouTube
Calculate the 'spin only' magnetic moment of M2+ (aq) ion (Z = 27). - Sarthaks eConnect | Largest Online Education Community